Small angle X-ray scattering (SAXS) is a powerful technique used to study the structure of materials at the nanoscale level. In recent years, it has become an essential characterization tool in numerous research fields such as materials science, biostructural research or renewable energy and storage. The technique became popular for a wide range of reasons including the fact that it does not require extensive sample preparation and it is non-destructive, which makes it fast and cost-effective. Although sample preparation is minimal, careful attention to detail when preparing for SAXS experiments is crucial to obtain high-quality data and accurate results.
In this article, we will discuss six common sample pitfalls that any researcher should look out for when preparing for SAXS experiments. By understanding these challenges and the strategies to bypass them, you can save time and resources, and ensure that your experiments yield reliable and reproducible results. Whether you are a seasoned SAXS user or new to the technique, this article will give you valuable insights that will help you get the most out of your experiments.
Advantages of SAXS sample preparation
Sample preparation is an essential aspect of any analytical technique including SAXS. However, in comparison with other laboratory characterization methods, the sample preparation for SAXS presents several noteworthy advantages:
- Minimal sample requirements: SAXS requires very small sample volumes, typically in the range of tens of microliters (smaller volumes down to a few microliters are possible depending on the sample environment). This makes it possible to analyze samples that are difficult or expensive to obtain in large quantities.
- Flexibility in sample preparation: SAXS can be used to study samples in solution or in the solid state, depending on the nature of the sample. Additionally, samples can be measured under a wide range of conditions, such as different temperatures, strains, or humidities, allowing for in situ analysis. Moreover, reactivity chambers and flow through measurements are available for monitoring chemical reactions.
- Simplicity of sample preparation: SAXS sample preparation is relatively simple and straightforward, with no special or complex preparation steps required. Samples can be analyzed as-is, with minimal or no modification, making the process faster and more cost-effective.
- Compatibility with a wide range of sample types: SAXS is a versatile technique that can be used to study a wide range of sample types, including polymers, nanoparticles, biological molecules, operando cells and more.
While SAXS sample preparation and data collection are rather straightforward, data analysis can be complicated, and it is thus important to ensure the absence of artifacts. Prior knowledge of the sample’s properties and behavior under various conditions can be crucial for conducting successful SAXS experiments. Here are some questions to ask yourself before starting:
1. How stable is my sample over time?
Some processes can cause a sample to become unstable, leading to changes in its structure and the SAXS scattering pattern. These processes may include:
- Aggregation or precipitation: They can occur in various types of samples such as proteins or nanoparticles and manifest as a change of size over time, which might be followed by sedimentation or flocculation. When aggregation takes place, a visual indication can be an increase in the turbidity of the solution. The formation of larger particles that scatter X-rays at different angles can lead to inaccurate results with increased sample size values and decreased porosity values. To avoid aggregation, it is important to ensure that the sample is well dispersed in the solvent and that the concentration is not too high.
- Sedimentation: Following sedimentation, a concentration gradient can form in the sample, which will induce alterations of the SAXS data. In addition, sedimentation can cause the sample to become more ordered or aligned along the vertical axis, which can affect the orientation and symmetry of the scattering pattern. To address sedimentation, lower concentration samples can be used while making sure to maintain the appropriate ratio between particles and surfactant. Another strategy to prevent sedimentation could be to use rotating capillaries and flow through sample cells. Fig. 1 shows an example of sedimentation occurring when the suspending media is not chosen correctly.

Fig. 1 1 µm Silica particles in various suspending media: (1) 100% water, (2) 50 % water 50 % isopropyl alcohol (IPA), (3) 100% IPA.
- Phase separation often occurs in unstable emulsions due to high concentrations of the sample components, incorrect choice of solvent or if the sample is not homogenous. New highly performant X-ray sources offer the possibility to perform SAXS measurements in rather short timeframes. However, as a rule of thumb to ensure reliable results, no changes in sample state (color/density) should be observed in a filled capillary over one hour.
2. Is my sample too absorbing?
Measurements performed on highly X-ray absorbing samples suffer from loss of signal and reduced sensitivity. This can however be bypassed by either using higher energy X-rays which are less likely to be absorbed compared to lower energy X-rays (their shorter wavelengths and higher frequencies allows them to penetrate deeper into the material without being absorbed) or by using a thinner sample. In the case of absorbing dispersions, diluting the sample and using thinner capillaries can also prove beneficial.
Fig. 2 (a) illustrates an example of the effect of the sample’s thickness on the scattered X-ray intensity. This shows that choosing an appropriate thickness is crucial for maximizing the scattering signal. It is however possible to estimate the adequate thickness prior to your experiments based on your sample’s characteristics. As can be observed in Fig. 2 (b) the transmission of X-rays decreases exponentially with respect to the sample’s thickness while scattering increases linearly with thickness.
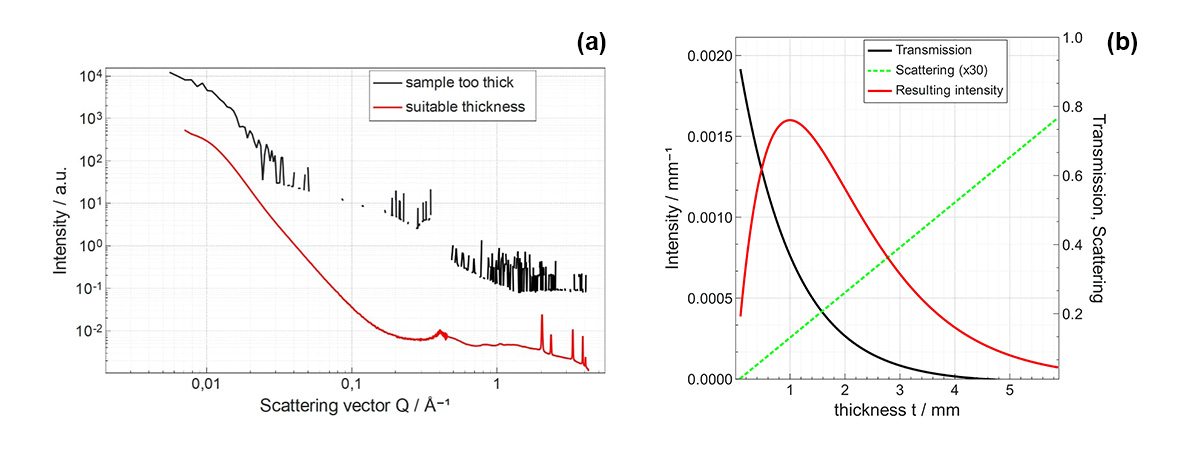
Fig. 2 (a) Metal-oxide powder SAXS/WAXS data sets obtained with different thicknesses using 8 keV radiation: (red curve) 61 % transmission and 2.1 µm apparent thickness and (black curve) 1.3 % transmission and 20 µm apparent thickness, data curves are shifted for clarity. The apparent thickness was determined from the measured transmission value using XSACT or CXRO [1]. (b) Evolution of water scattered intensity as a function of sample thickness (in red) plotted along the transmission at 8 keV (in black) and scattering (in green) curves. The transmission curve highlights the exponential attenuation relationship exp(-µt) where t is the sample’s thickness and µ is the linear attenuation coefficient. Maximum scattered intensity is then found at a thickness equal to 1/µ
In numerical terms, it is generally advised that the transmission should not drop below 10% for adequate measurement quality. A value around 36% is considered optimal as a rule of thumb, lower values of transmission increase the likelihood of multiple scattering events, while higher values result in reduced signal.
Finally, an heavily absorbing sample is potentially much prone to multiple scattering [2]. It is therefore encouraged to reduce thickness of sample to remain in the optimum transmission value enabling simplified analysis of the scattering process.
3. Why is my sample not scattering?
Sometimes it can happen that your sample is not producing a SAXS scattering pattern. This however does not necessarily imply a lack of structural information. There are various factors that can contribute to the absence of scattering in SAXS experiments:
Insufficient Contrast: SAXS relies on the scattering contrast between different components or structures within the sample. If the scattering contrast is low, meaning the materials have similar electron densities or compositions, the scattering signal may be weak or undetectable. The difference in scattering length density between the sample and its matrix should be larger than 0.01%.
To increase the scattering contrast and improve the visibility of certain features or components during SAXS experiments contrast enhancement techniques using ethanol (EtOH) can be used. EtOH has a relatively high atomic number making it a strong X-ray scatterer. By adding it to a sample, the scattering from ethanol atoms can be significantly enhanced compared to the surrounding materials, thereby increasing the contrast in the SAXS patterns.
This technique is particularly useful when studying samples with low inherent scattering contrast, such as biological macromolecules or soft matter systems.
Fig. 3 illustrates the impact the choice of the suspending media can have on the data collected during SAXS experiments. The plot shows simulated SAXS data collected from a solution of polystyrene latex hard spheres with diameters of 100 nm and concentration of 1mg/ml using different solvents: water, ethanol and a 1:1 mixture of water and ethanol. While using pure water as suspending media will produce a signal sufficient to extract various morphological parameters, such as the particle diameter, it is clear that, using pure ethanol and even a mixture of the 2 solvents provides better contrast. This greatly increases the data quality resulting in a more reliable analysis.
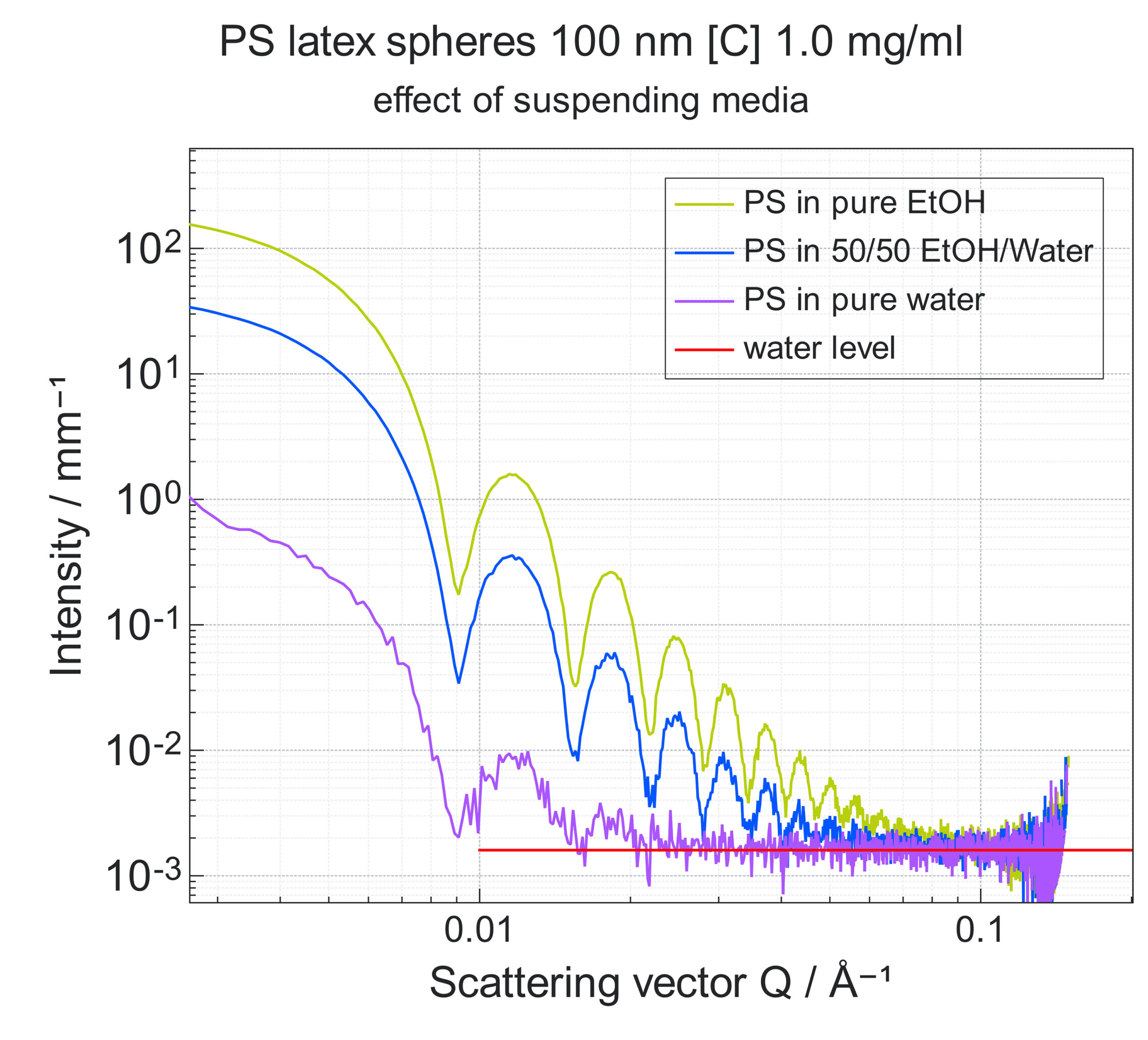
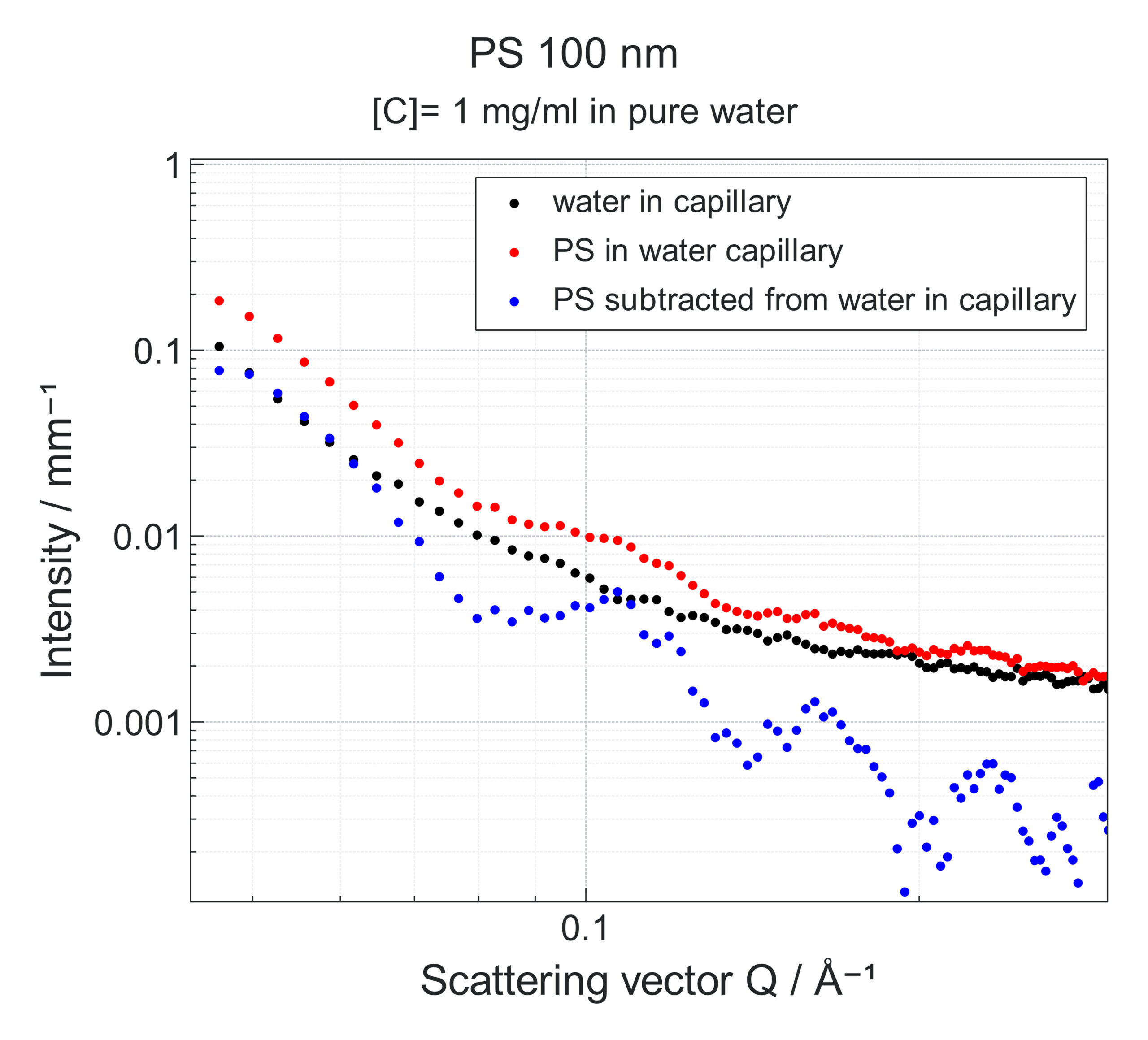
Fig. 3 Simulated SAXS data collected on a Xeuss 3.0 instrument at a 1.8 m detector distance. The water signal was not subtracted from the data. The water intensity level is marked in red. The simulation was performed using the XSACT software.
Fig.4 Experimental SAXS data obtained on a Xeuss system from a solution of polystyrene spheres of 100 nm diameter and concentration of 1mg/ml in water (red curve), or from a water solution only (black curve). Blue curve is the subtracted data enabling to extract scattering signal from the polystyrene spheres for further size and shape analysis.
Another important step to consider when performing the SAXS measurements is to record the scattering of the buffer without the sample. By subtracting it from the scattering signal of the sample mixed with the buffer one can isolate the sample contribution to the intensity making for a more precise analysis of the colloid’s morphological parameters. Fig. 4 shows an example of the impact that buffer subtraction can have on the data analysis and interpretation.
Sample Concentration: Extremely dilute samples may not exhibit significant scattering due to the low density of scattering centers. Conversely, highly concentrated samples can experience particle interactions that may also lead to aggregation. In presence of particle interactions the scattering intensity will be composed of a structure factor (describing the interactions) and a form factor (describing the particles themselves). To best study the form factor, aim would be to maximize the concentration without entering into regime where particles are interacting. Conversely structure factor could be extracted from a concentrated suspension by applying a division with the form factor previously determined from a diluted suspension of given sample, provided measurements are performed in absolute intensity (for example see equation 22 of [3])
4. Is my sample flat and free from irregularities?
If you are interested in using grazing incidence small and wide angle X-ray scattering (GISAXS or GIWAXS) to investigate the structural properties of thin films and surfaces, sample flatness is of utmost importance to ensure the reliability and interpretability of the results. A flat sample ensures a consistent grazing angle across the entire surface, allowing for precise Q-value calculations. This minimizes at the same time specular reflection artifacts (interference of the direct reflection of the incident X-ray with the scattered signal), which can cause background noise and obstruction of the desired scattering features. Additionally, sample flatness helps suppress multiple scattering events by reducing the chances of the X-ray beam encountering irregularities or variations in the sample thickness, thereby providing a cleaner scattering pattern.
To minimize the effect of surface irregularities silicon wafers are routinely used as sample support, ensuring atomic-scale flatness.
5. Is my sample heterogeneous?
Sample heterogeneity, particularly in composites, can have significant effects during SAXS experiments. In composite materials such as reinforced rubber or polymers, the presence of different components with varying scattering properties can result in complex scattering patterns composed of a superposition of individual scattering contributions. This can make the interpretation and analysis of SAXS data more challenging.
Another type of inhomogeneity is polydispersity: samples with varying crystallite or particle sizes, that will exhibit broadened peaks or smeared oscillations. This broadening arises from the variation in scattering length scales across the sample, leading to a distribution of scattering angles. As a result, the peaks become wider and less well-defined, making it difficult to extract precise structural information.
Thirdly, thickness variations as well as packing density and distribution of the powder particles can also have significant influences on the scattering pattern. Variations in sample thickness across the measurement area can lead to uneven X-ray absorption and scattering, resulting in intensity variations and distortions in the scattering pattern.
Achieving a homogeneous distribution of components throughout the sample can help minimize variations in scattering behavior and improve data quality. Proper sample preparation becomes thus crucial for heterogeneous samples to ensure representative measurements. For this, X-ray mapping and imaging can play a valuable role. X-ray mapping involves scanning a focused X-ray beam over the sample surface and collecting the emitted X-rays. This allows for the construction of maps that illustrate the distribution and variation of certain properties extractable from SAXS such as crystallinity, thickness, lamellar spacing, degree of lamellar orientation or the primary orientation of the lamellae. X-ray imaging can also reveal the presence of inhomogeneities, phase separations or concentration gradients within the sample. The integration of SAXS and radiography analysis provides valuable insights into the relationship between macrostructural heterogeneities and the nano- or atomic structure of heterogeneous samples.
6. Did I choose the right sample holder for my system?
Competition between the scattering from the sample itself and the scattering from the sample holder or support material can arise during SAXS experiments. To minimize the impact of sample holder scattering, it is thus important to choose an appropriate sample holder material, optimize the sample holder design, but also implement proper background subtraction techniques. For instance, it can be difficult to measure a plastic sample of 0.1 mm thickness placed between 2 foils of 0.1 mm of Kapton which is also a plastic. As such typical Kapton foil thicknesses are chosen to be significantly thinner than sample.
7. Can my sample suffer from radiation damage? (Bonus question)
When using laboratory X-ray sources radiation sensitivity is generally not a significant problem. Here is thus one thing less to worry about. The extent of radiation damage to a sample can however vary, depending on its specific characteristics and composition. To mitigate the effects of radiation damage, researchers can adjust the duration of the experiment.
In this article, we have explored six common sample pitfalls that researchers should be mindful of when conducting SAXS experiments. By being aware of these challenges and employing appropriate strategies to overcome them, one can optimize the experimental setups, save valuable time and resources, and obtain reliable and reproducible results.
[1] The Center for X-Ray Optics within Lawrence Berkeley National Laboratory, X-Ray Interactions with Matter: https://henke.lbl.gov/optical_constants/filter2.html.
[2] E. F. Semeraro, J. Möller, and T. Narayanan, Multiple-Scattering Effects in SAXS and XPCS Measurements in the Ultra-Small-Angle Region, J Appl Cryst 51, 706 (2018). DOI: 10.1107/S160057671800417X
[3] in “Soft Matter Characterization, R. Borsali & R. Pecora, Springer 2008. ISBN 978-1-4020-4464-9 – DOI: 10.1007/978-1-4020-4465-6”. Chapter: Synchrotron Small Angle Scattering, pp 899 – 952 by T. Narayanan,
Products
Discover our SAXS instruments in the lab
Xeuss Pro
The Ultimate Solution for Nanoscale Characterization using SAXS/WAXS/GISAXS/USAXS/Imaging


































