WAXS measurements reveal that the crystalline structure of silk is preserved by gentle degumming
Silks are well known natural protein fibers, spun by spiders, silkworms and other insects, that have been used for centuries in the textile industry due to their high strength and toughness. In particular, the silk of the domestic silk moth Bombyx mori (B. mori) has been intensively studied for its potential applicability as a biomedical material [1, 2]. Silk contains two proteinous polymers [3] (shown in Fig. 1):
- Fibroin: composed a of hydrophobic heavy chain (FibH) and hydrophilic light chain (FibL). The dominant FibH itself consists of large repetitive units of alanine (ALA) and glycine (GLY) that fold into antiparallel – sheet crystalline structures which in turn are organized in a hydrophilic amorphous matrix formed by FibL.
- Sericin: an antigenic gum-like hydrophilic protein, forming the outer coating that keeps the fibroin fibrils together.
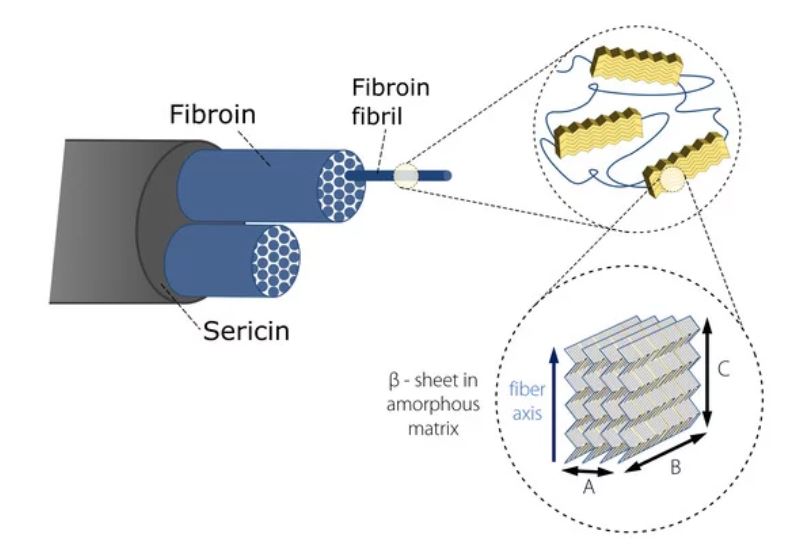
Figure 1. Hierarchical structure of Bombyx Mori silk (representation inspired by [3, 4]).
In this application note, WAXS measurements, performed on thin single fibers (approximately 25 μm and 20 μm fiber diameter in raw and degummed state respectively as measured by scanning electron microscopy (SEM) see inset of Figs. 2 (a) and (b)), have been used to study the effect of degumming on the crystalline structure of silk.


































