- Application highlight -
These days, batteries are ever-present making it difficult to picture modern life without them. Worldwide the rechargeable batteries market value has seen a rapid increase over the last decades driven by the global awareness of environmental protection, the expanding electric vehicle market, and the support shown by governments around the world [1]. In particular, lithium-ion batteries have seen the highest growth [2] and became the most commonly used rechargeable batteries owing to their cost advantages and superior technical performance (highest energy and power density, smaller and lighter than other rechargeable batteries, high number of charge and discharge cycles in the battery’s life) [1]. The lithium output, however, may not be able to catch up with demand from battery producers which is expected to more than double over the next decade as global electric vehicle adoption continues to rise. This discrepancy has already manifested as a surge in the prices of raw materials, battery-grade lithium carbonate in particular, over the last couple of years [3].
It is thus imperative to advance the understanding and development of alternative battery chemistry in order to counteract the global reliance on one type of technology and the depletion of critical materials. Sodium-ion batteries (NIBs) are considered the most promising large-scale energy storage alternative due to the natural abundance and widespread distribution of raw materials, inexpensiveness, and superior materials sustainability in comparison with lithium-based technologies [4].
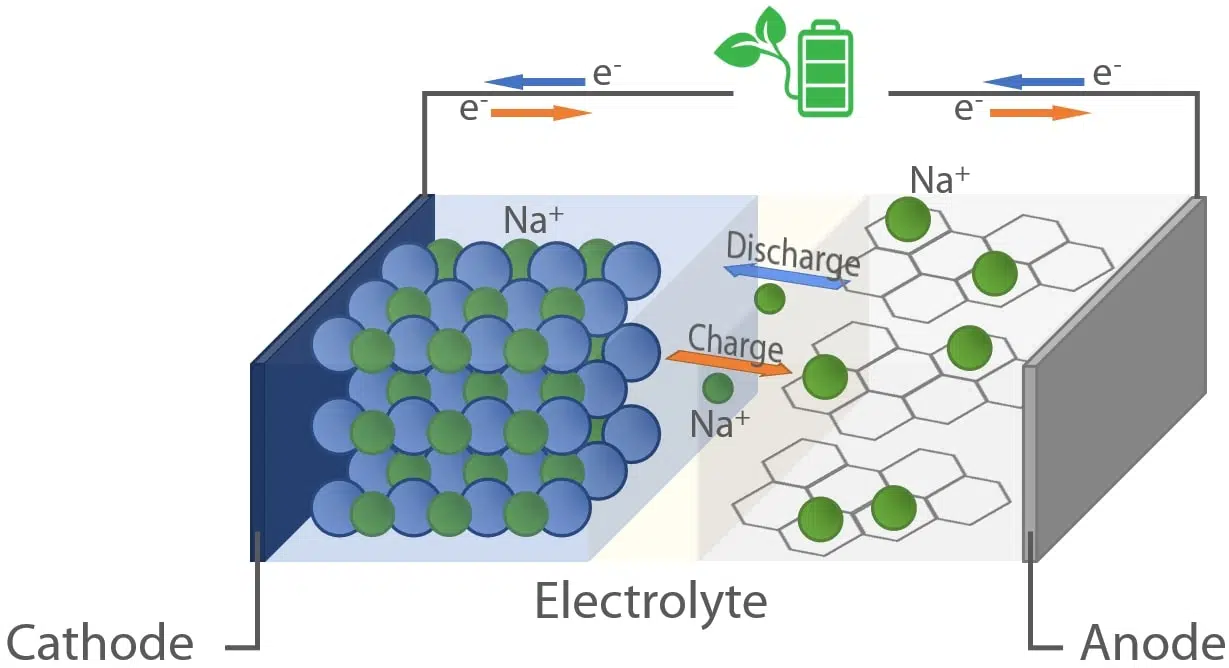
Fig. 1 Schematic representation of a Na-ion battery cell.
The working principle and cell design of NIBs are analogous to the operating mode of lithium-ion batteries with the fundamental difference that the charge carriers are sodium ions (Na+). As depicted in Figure 1, a rocking chair mechanism is employed to store energy by converting chemical and electrical energy into each other. During charging, electrical energy is used to promote the movement of electrons from the cathode to the anode. At the same time, charge neutrality is maintained by the release of sodium ions from the cathode and their migration to the anode through the electrolyte. The reverse process occurs during the discharge.
However, from a design perspective, Na has a much larger ionic radius (1.02 Å) than Li (0.76 Å) making it challenging to find appropriate anode materials for hosting the Na+ ions that allow at the same time a rapid insertion and de-insertion. As the most used anode material in lithium-ion batteries, graphite, does not support sodium intercalation, a considerable amount of research has focused on developing other suitable electrodes. Owing to their high abundance and low costs, carbon-based anode materials remain the most promising systems for NIBs as well [5]. In order to obtain a high reversible capacity, most designs focus on creating anode materials with a substantial concentration of micropores accessible only to Na+ ions. Sieving carbons (featuring highly tunable nanopores with tightened pore entrance) [6], biomass-derived hard carbon [7] or hard carbons with a tailored pore network [8] are only a few examples of proposed high-energy carbon anodes.
It is thus essential to study the pores network to understand and maximize sodium ion storage. For this, small angle X-ray scattering (SAXS) is an excellent probing technique that can be used to determine the pore-size and pore-size distribution of both open and closed pores, information that is not available by using other techniques such as gas absorption analysis. Additionally, the filling of the pores can be monitored through SAXS measurements.
Sieving carbons
It has previously been shown that porous carbons (PC) are unsuitable anodes materials for sodium ion batteries as their large surface area pores are also accessible to electrolytes which in turn induced the formation of an undesired solid electrolyte interphase (SEI) inside the nanopores [9]. To solve this issue, sieving carbons (SC) with tightened pore entrance (< 0.4 nm) have been shown to exhibit a superior electrochemical performance [6]. SAXS measurements have revealed that the filling of the pores with SEI can be prevented by depositing methane on commercial PC which helps tighten the pore entrance while at the same time maintaining a similar pore surface area and body diameter. As shown in Figure 2, the nanopores SAXS signal of SC is invariant during cycling while the PC signal shows the disappearance of a broad peak in the intermediate q range pointing towards a filling of the pores with SEI.
In terms of electrochemical performance sieving carbons greatly surpass porous materials. At 50 mA/g current density PC show only a 39 mAh/g reversible capacity while SC exhibit a much higher reversible capacity of 328 mAh/g.
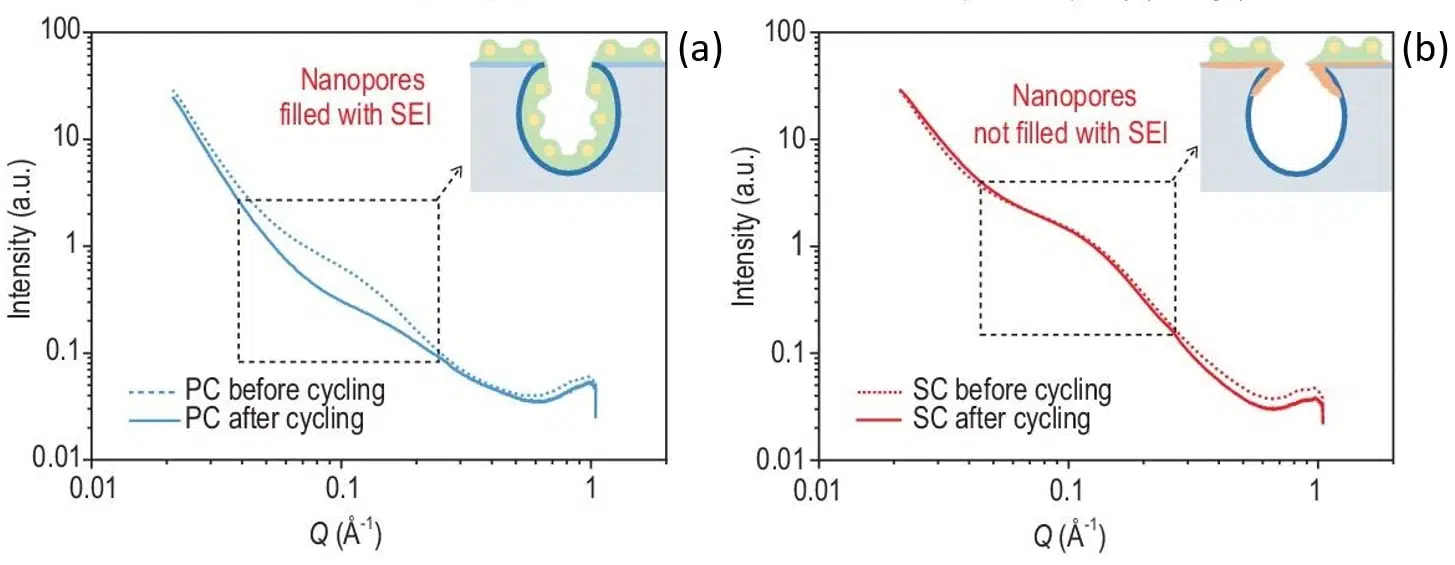
Fig. 2 SAXS patterns of (a) porous carbon and (b) sieving carbon anodes recorded before and after 5 cycles at a current density of 50 mA/g. The insets display the tightened pore entrance of SC (orange) together with a representation of the solid electrolyte interphase (SEI) distribution (green) with respect to the pores. Credit: National Science Review, 2022, DOI: 10.1093/nsr/nwac084.
Hard carbons with a tailored bimodal pore network
Tunning the texture of carbons is another strategy that has been proposed to maximize their performance as anodes for NIBs [8]. Hard carbons with a bimodal pore network composed of internal micropores interconnected through mesopores have been solvothermally prepared. The second mesoporous network aims to enhance the connectivity between micropores thus facilitating the Na+ diffusion and ultimately increasing the capacity of the battery.
SAXS measurements have shown that the size of both micro and mesopores can be tuned by varying the pyrolysis temperature. As displayed in Figure 3 (a), higher temperatures produce pores of larger diameters. This increase in size has been subsequently correlated with a superior capacity retention as it can be deduced from Figure 3 (b).
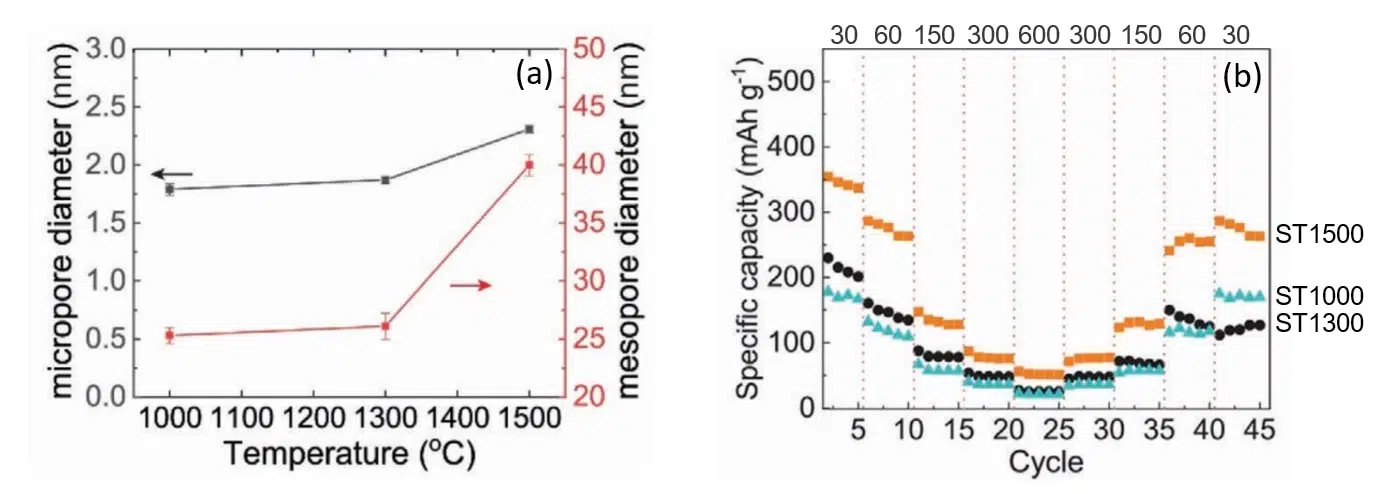
Fig. 3 (a) Micro and mesopores diameters as a function of pyrolysis temperature as determined by SAXS. (b) Rate performance of discharge capacities at various current densities (indicated in the upper part of the figure in units of mA/g) for carbon anodes pyrolyzed at 1000, 1300 and 1500 oC. Credit: Advanced materials interfaces, 2022, DOI: 10.1002/admi.202101267.
Biomass-derived hard carbon
Hard carbons derived from biomass have received considerable attention as they would increase the sustainability of battery materials. Besides being cost-effective, abundant and easily accessible these materials often display a suitable macro and micro-architecture and scalable manufacturing methods. Rosewood, a by-product of furniture industry, for example is a naturally porous material which shows great potential as anode material for sodium-ion batteries [7].
Through a simple chemical treatment part of the lignin and the hemicellulose can be removed. After calcination at a relatively low temperature (1100 oC), hard carbons with an increased number of closed pores and reduced wall thickness (which favors Na+ diffusion) can be obtained. SAXS measurements proved that chemically treated samples (ChT-1100) contain a higher number of closed pores than the untreated parent compound (UnT-1100). As visible in Figure 4 (a) the SAXS pattern of ChT-1100 exhibits a more pronounced plateau in the intermediate q region indicative of a higher pore content.
In terms of electrochemical performance, it is obvious from Figure 4 (b) that chemically treated materials display a significantly improved reversible capacity.
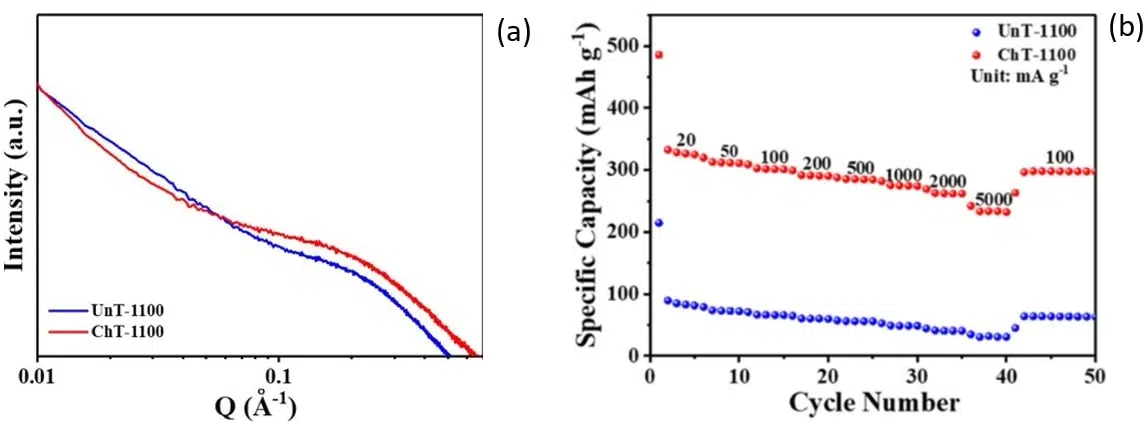
Fig. 4 (a) SAXS patterns of chemically treated (ChT-1100) and untreated (UnT-1100) rosewood samples. (b) Rate performance of discharge capacities at various current densities (indicated in the figure in units of mA/g) for chemically treated and untreated rosewood samples. Credit: SusMat, 2022, DOI: 10.1002/sus2.60.
Conclusions
The development of efficient anode materials is a crucial step in optimizing the performance of sodium-ion batteries. The work presented here highlights the potential of several carbon-based anode materials to provide good sodium storage and high reversible capacities. As the closed pores are the main microstructure accommodating Na+ ions, understanding and ultimately designing an appropriate pore architecture is key to producing high-energy carbon anodes.
In this respect, SAXS has proven extremely helpful in characterizing the pore structure revealing the size and amount of pores as well as their state: filled or empty.
The research was originally published in the following articles:
[6] Li, Qi, Xiangsi Liu, Ying Tao, Jianxing Huang, Jun Zhang, Chunpeng Yang, Yibo Zhang et al. “Sieving carbons promise practical anodes with extensible low-potential plateaus for sodium batteries.” National Science Review (2022).
[7] Zhou, Siyu, Zheng Tang, Zhiyi Pan, Yuancheng Huang, Le Zhao, Xi Zhang, Dan Sun, Yougen Tang, Abdelghaffar S. Dhmees, and Haiyan Wang. “Regulating closed pore structure enables significantly improved sodium storage for hard carbon pyrolyzing at relatively low temperature.” SusMat (2022).
[8] Alptekin, Hande, Heather Au, Emilia Olsson, Jonathon Cottom, Anders CS Jensen, Thomas F. Headen, Qiong Cai, Alan J. Drew, Maria Crespo Ribadeneyra, and Maria‐Magdalena Titirici. “Elucidation of the Solid Electrolyte Interphase Formation Mechanism in Micro‐Mesoporous Hard‐Carbon Anodes.” Advanced materials interfaces 9, no. 8 (2022): 2101267.
[1] Rachid Amui, Janvier Nkurunziza, COMMODITIES AT A GLANCE Special issue on strategic battery raw materials. United Nations Conference on Trade and Development, 2020.
[2] Pillot Christophe, The Rechargeable Battery Market and Main Trends 2018-2030. AVICENNE ENERGY, 2019.
[3] Fitri Wulandari, Lithium price forecast: Will the price keep its bull run?, Capital, July 9, 2022, https://capital.com/lithium-price-forecast.
[4] Alptekin, Hande, Heather Au, Emilia Olsson, Jonathon Cottom, Anders CS Jensen, Thomas F. Headen, Qiong Cai, Alan J. Drew, Maria Crespo Ribadeneyra, and Maria‐Magdalena Titirici. “Elucidation of the Solid Electrolyte Interphase Formation Mechanism in Micro‐Mesoporous Hard‐Carbon Anodes.” Advanced materials interfaces 9, no. 8 (2022): 2101267.
[5] Palaniyandy, Nithyadharseni, and Mesfin A. Kebede. “Sodium-ion battery anode materials and its future prospects and challenges.” Electrochemical Devices for Energy Storage Applications (2019): 41-58.
[6] Li, Qi, Xiangsi Liu, Ying Tao, Jianxing Huang, Jun Zhang, Chunpeng Yang, Yibo Zhang et al. “Sieving carbons promise practical anodes with extensible low-potential plateaus for sodium batteries.” National Science Review (2022).
[7] Zhou, Siyu, Zheng Tang, Zhiyi Pan, Yuancheng Huang, Le Zhao, Xi Zhang, Dan Sun, Yougen Tang, Abdelghaffar S. Dhmees, and Haiyan Wang. “Regulating closed pore structure enables significantly improved sodium storage for hard carbon pyrolyzing at relatively low temperature.” SusMat (2022).
[8] Alptekin, Hande, Heather Au, Emilia Olsson, Jonathon Cottom, Anders CS Jensen, Thomas F. Headen, Qiong Cai, Alan J. Drew, Maria Crespo Ribadeneyra, and Maria‐Magdalena Titirici. “Elucidation of the Solid Electrolyte Interphase Formation Mechanism in Micro‐Mesoporous Hard‐Carbon Anodes.” Advanced materials interfaces 9, no. 8 (2022): 2101267.
[9] Li, Kaikai, Jun Zhang, Dongmei Lin, Da-Wei Wang, Baohua Li, Wei Lv, Sheng Sun et al. “Evolution of the electrochemical interface in sodium ion batteries with ether electrolytes.” Nature communications 10, no. 1 (2019): 1-10.

































