Hierarchical self-assembly of small molecules is a tried and tested method to obtain nanostructured materials. The intrinsic dynamics and (bio)degradability of these materials, however, limit their application to use as biomaterials. For many other applications the lack of stability restricts their suitability. In a recent publication in Nature Nanotechnology, scientists from Massachusetts Institute of Technology elegantly use the benefits of self-assembly while circumventing instability issues by using aramid amphiphiles[1]. These amphiphiles undergo spontaneous self-assembly in water to form high-aspect-ratio structures. The formed nanoribbons are intrinsically resistant to hydrolysis because the hydrogen bonds, which form the self-assembled structure, are buried in the hydrophobic interior.
With the developed approach, the scientists from MIT did produce dried bundle of nanoribbons as a first demonstration of capability for solid state materials synthesis. The flexible dried threads were produced in two steps. First, a one-dimensional gel is made by annealing the ribbons in water and pulling the resulting suspension through a salt solution. In the second step, the gel is dried, leading to the formation of a 20 µm wide thread. Laboratory Small and Wide-Angle X-ray Scattering (SAXS and WAXS) instrument was used to investigate the aligned nanoribbons threads.
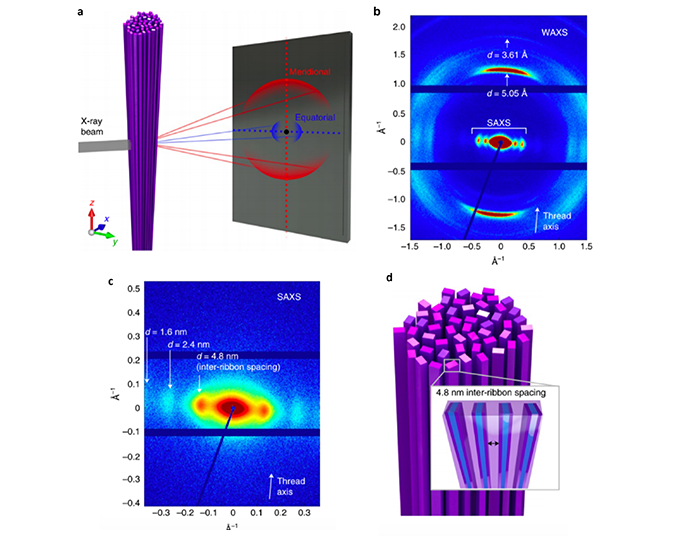
a) Orientation of a solid-state nanoribbon thread with meridional and equatorial scattering on the X-ray detector; b) 2D WAXS pattern; c) 2D SAXS pattern; d) Hypothesized structure showing the alignment of individual nanoribbons.
Credit: images extracted from Nat. Nanotechnol. 2021, doi 10.1038/s41565-020-00840-w
WAXS measurements on the thread (a) confirm that the nanoribbon structure remains intact throughout the formation and drying. This is most clearly seen by the presence of peaks in the meridional direction corresponding to the intermolecular distance and π-π stacking of the aramid molecules (b). The peaks in the WAXS-regime imply that intermolecular aramid hydrogen bonding is dominant along the long axis of the nanoribbon. Zooming in into the SAXS-regime (c) shows peaks in the equatorial direction, these equidistant peaks are indicative of 4.8 nm spacings between nanoribbons.
In addition to the characterization of the final dried state of the nanoribbons, X-ray scattering can be used to quickly assess the self-assembly structure in solution at earlier synthesis stage and including at various temperatures by probing simultaneously the organized molecular packing (WAXS) and long hierarchical order (SAXS).
































