Cosmetic products, such as for example hair conditioners, have to meet a high number of requirements in order to fulfill the customer’s needs. Stability, a pleasant odor and look, a creamy texture and the capability to modify the hydrophilic surface properties of the hair are some of the most important examples. Small amounts long chain alcohols and cationic surfactants can form swollen bilayers that trap high amounts of water under the right processing conditions. These gel networks consist mainly of multilamellar vesicles (MLVs), the vesicle walls are formed of Lb lipid bilayers consisting of hexagonally packed alcohol and surfactant molecules. This gel network of multilamellar vesicles is responsible for the creamy texture of the conditioner.
Even though the cooling rate has long been known to play an important factor in the production of gels based on long-chain alcohols and surfactants, the physical-chemical cause for these differences remained elusive. Cetearyl alcohol and cetyltrimethylammonium chloride (CTAC) form the basis of many pharmaceutical and cosmetic formulations. In a recent study,1 researchers from the chemical department of the University of Bari (Italy) in collaboration with L’Oreal and Lund University (Sweden), shed a light on the link between the cooling process and the rheological properties of the resulting gel. Using a multi-technique approach, they found that the use of different cooling rates results in the formation of multilamellar vesicles with different repeat distances. The gels formed with different processes show significantly different elastic and viscous moduli.
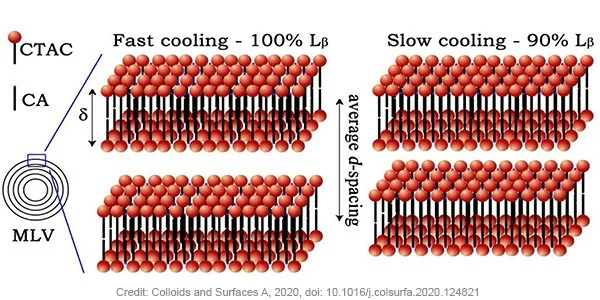
Gel samples consisting 5 wt% of cetearyl alcohol and 6 wt% of CTAC were prepared by heating up to 85 ˚C. The sample was either quenched on ice, or left to cool to room temperature in air. The quenched gel showed elastic (G’) and viscous (G’’) moduli of around four times higher than the gel that was left to relax in air, hereby influencing the spreadability and the feel of the gel. Small-Angle X-ray Scattering (SAXS) measurements of both samples confirmed the presence of multilamellar vesicles. Kratky plot analysis revealed a difference in the interlamellar d-spacing of the bilayers of the two samples, 31.4 nm was found for the quenched sample and 28.5 nm for the relaxed sample. Comparing with theorical values from Lβ phase this reveals that the quenched sample consists of fully swollen Lβ phase while relaxed sample is a multi-phase gel network composed of mainly Lβ phase. Using the lipid bilayer form factor, the scattering length density was fitted and a similar bilayer thickness of 3.8 nm (δ) was extracted for both samples. The bilayer thickness combination with the average d-spacing for both samples allow to calculate the volume fraction of cetearyl alcohol and CTAC inside vesicles to be 0.83 for the quenched sample and 0.77 for the relaxed sample. In other words, in the relaxed sample, a larger volume fraction of the alcohol and surfactant form lipid bilayers that are not incorporated in vesicles. This has an influence on the average bending rigidity which is higher for the quenched sample.
In summary this study shows that although fast and slow cooling both result in the formation of multilayered vesicles, there is a difference in the amount of material incorporated into the vesicles as well as a difference in swelling of layers. These differences result in different bending rigidity and different rheological properties. Understanding these parameters helps in the preparation of complex pharmaceutical and cosmetic formulations with desired thickness, richness of feel and spreadability.
































