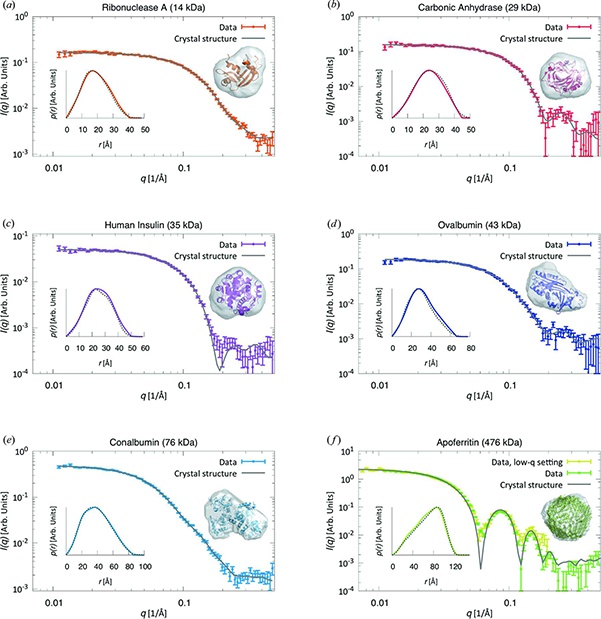
A major increase of productivity in biostructural research is achieved by eliminating the need to go to a synchrotron for the combination of SAXS with SEC (size exclusion chromatograpy). In a recent publication in the Journal of Applied Crystallography Saskia Bucciarelli (group of Bente Vestergaard) and collaborators at the University of Copenhagen show how they obtained structural details of water soluble proteins using this combination of techniques. The use of SEC-SAXS is a very well-known method at synchrotron beamlines. It enables researchers to analyze the structure of very delicate samples such as labile proteins and complexes with low binding affinities that would fall apart in storage or during transport to the synchrotron.
In a collaboration with Xenocs, the researchers demonstrate that for a number of different proteins, SEC-SAXS allows them to obtain data that has sufficiently high quality to extract structural information – just like at the synchrotron! Simultaneous with the collection of the SAXS pattern, UV-Vis absorption is measured, enabling accurate measurement of the concentration of the sample, which in turn is essential for determination of the molecular weight.
SEC-SAXS in the lab does not only give results comparable to synchrotron data, it actually has an additional benefit! At the synchrotron radiation damage often, if not always, destroys the sample. In the lab this is not a problem, so the scientists can collect the sample after the measurements and run additional analyses on it.
http://journals.iucr.org/j/issues/2018/06/00/vg5091/index.html