To combat climate change, the use of renewable energies is regarded as one of the top priority actions to be implemented both by industries as well as private individuals. Today the use of so-called clean energy is limited by several factors amongst which its intermittent nature, with power fluctuating across a variety of time frames, being a particularly challenging one. Moreover, the limited efficiency of the widespread lithium batteries hinders the transition to zero-emissions sources of electricity [1]. Alternatively, fuel cells evade the time-dependent generation, transport and storage of electrical energy issues by employing a chemical reaction to convert an energy source into electricity. In particular, proton exchange membrane fuel cells (PEMFCs) are regarded as promising renewable power sources for transportation and other mobile applications [2].
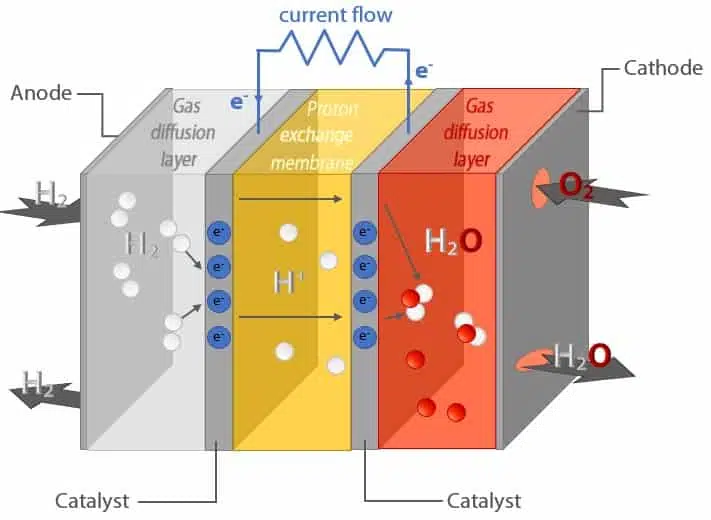
Fig. 1 Schematic representation of a proton exchange membrane fuel cell. Two chemical reactions take place at the anode ( 2H2 -> 4H+ + 4e–) and cathode (O2 + 4H+ + 4e– -> 2H2O) consequently generating a current flow.
A brief introduction to degradation processes of carbon-supported platinum electrocatalysts
To catalyze both the oxygen reduction and the hydrogen oxidation reactions, carbon-supported platinum or platinum alloy nanoparticles are currently the most exploited electrocatalysts for PEMFCs. However, the high cost and scarcity of Pt hinder large-scale commercialization. To make this type of fuel cells competitive for practical applications, a great amount of research focuses nowadays on understanding the degradation mechanisms with the ultimate goal of increasing the catalytic activity and stability. Accelerated stress tests (ASTs) are routinely employed to reduce the testing time during experiments. As a function of applied potential, two degradation paths have been distinguished [3]:
- for operation potentials between ≈ 6 and ≈ 1 V: electrochemical Ostwald ripening will favor the formation of larger Pt nanoparticles at the expense of smaller ones.
- for excess cathode potentials of ≈ 5 V: the carbon support’s corrosion will induce the detachment and ultimately the coalescence of Pt nanoparticles.
The study of particle size distribution at the nanoscale becomes thus an essential part of the research aiming to understand the degradation of electrocatalyst materials. For this purpose, small angle X-ray scattering (SAXS) is a powerful technique that can offer valuable information about the morphology of the catalyst (shape, size and size distribution of nanoparticles). Time-resolved evolution of particle size distribution during the catalyst degradation cycle can be inferred from either ex-situ measurements performed on a large number of samples or through more suitable in-situ or operando measurements. Operando SAXS experiments are typically performed using synchrotron X-ray sources owning to their intense beams and straightforward background subtraction of the carbon support [3]. For measurements performed on laboratory sources, the background is usually measured ex-situ on a platinum-free carbon electrode subjected to the same degradation process as the studied one. Typically, this requires a secondary separate experiment which can give rise to additional errors in the normalization process if the measuring conditions are not the same [4].
Advances in data acquisition offer the possibility to record high-quality operando SAXS data of catalyst degradation with a laboratory X-ray source
Recently, a group of researchers from the University of Copenhagen and University of Bern [5] has proposed a new design for the working electrode which allows the recording of the operando background data on a catalyst-free section of the cell which receives the same electrochemical treatment as the catalyst film. Their proof-of-concept study showed that proper background subtraction can be obtained for operando SAXS measurements performed on laboratory X-ray sources as a part of a single experiment.
Probability density functions obtained using background data collected before and after the accelerate stress tests (i.e. with the newly designed working cell) are compared to data recorded on a pristine electrolyte-free sample measured ex-situ (see the figure below). The first observation noted is that normalisation to the background scans recorded after the electrochemical test (Fig. 2 b) produces a better agreement with the pristine sample than normalisation to background scans recorded before the electrochemical test (Fig. 2 a).
Furthermore, when analysing the particle size distribution as a function of AST steps, using a model comprising two distinguishable size particle populations, two transformations become evident as depicted in Fig. 2 (b):
- the size of particles belonging to the small particles population increases continuously while the size of large particles remains constant.
- the probability density of small particles decreases concomitant with an increase of the probability density of large particles.
It is thus concluded that operando SAXS measurements reveal that, when subjected to ASTs, the degradation of the Pt/C fuel cell consists of a continuous dissolution of Pt step followed by Oswald ripening in contrast with the alternative scenario of particle coalescence.
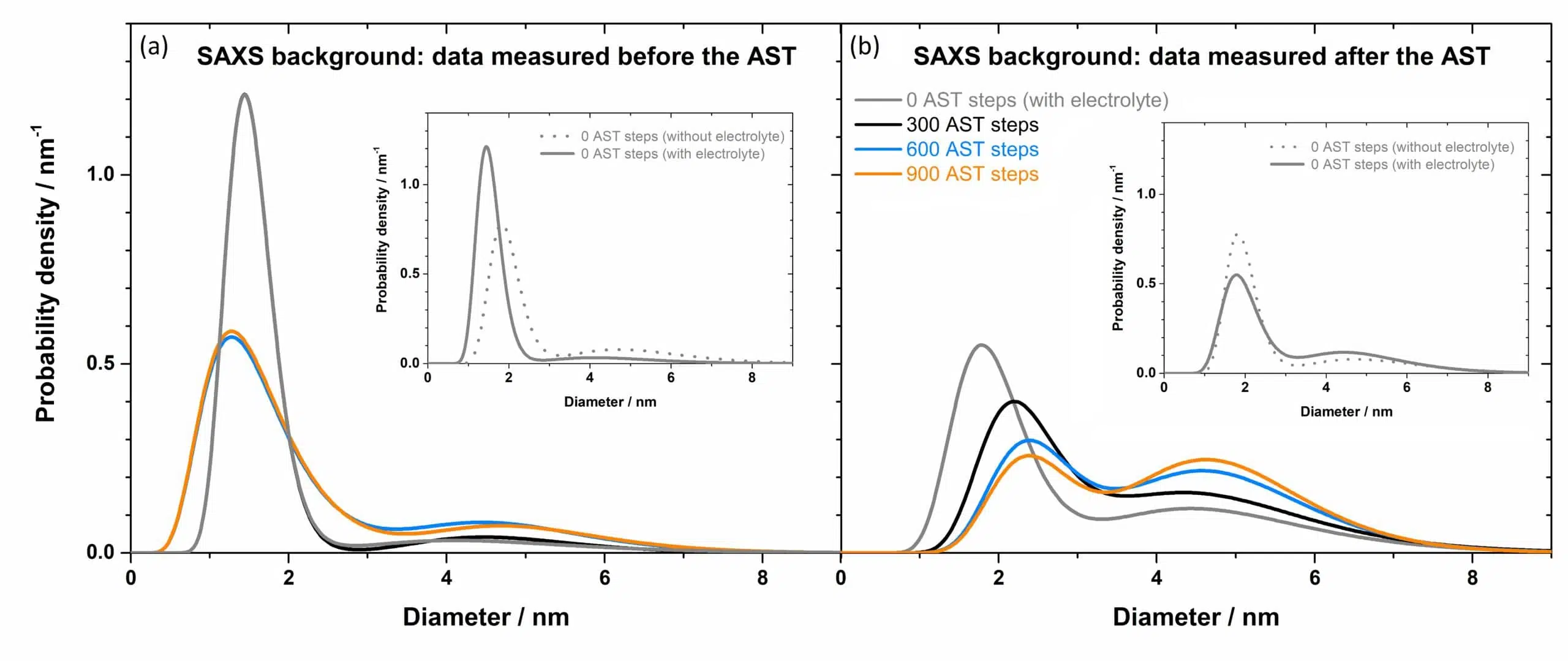
Fig. 2 Probability density as a function of particle diameter, as obtained from SAXS measurements, recorded at various instants during the AST cycle. (a) Data normalized to background scans recorded before the AST cycle. (b) Data normalized to background scans recorded after the AST cycle. The inset of each figure depicts the comparison between the sample before applying the AST cycle (and normalized to its respective background scan) and a pristine ex situ sample (measured without electrolyte). Courtesy of Johanna Schröder and Jacob J. K. Kirkensgaard.
This proof-of-concept study demonstrates the possibility of acquiring high-quality operando SAXS data of catalyst degradation with a laboratory X-ray source and a modified electrochemical cell. The advancements in background subtraction open the door to in-house measurements that offer more flexibility for experiment optimization and reproducibility studies compared to synchrotron beamtimes. At the same time, preliminary SAXS data obtained in a laboratory setting can be used to design more efficient synchrotron experiments.

































